The power of our portfolio.

Accelerating Translational Innovation from Discovery to Market.
Genovyn integrates world-class scientific discovery, preclinical development, and global clinical execution into a unified biotechnology platform. We combine advanced discovery and preclinical capabilities with Genovyn’s leadership-driven expertise in clinical operations, commercialization, and strategic development.
Powered by Today’s best technology. And tomorrow’s.
Our scientific foundation is anchored by the proven capabilities of our leadership team, with global reach for precision medicine, translational biology, and IND-enabling research.
- Core Capabilities Target & Lead Discovery – AI-driven target identification, hit-to-lead optimization, and medicinal chemistry integrated with in-silico modeling and SAR analysis.
- In Vitro / In Vivo Characterization – Robust ADMET profiling, pharmacokinetic and pharmacodynamic studies, efficacy modeling, and biomarker correlation.
- Toxicology & IND-Enabling Studies – Comprehensive preclinical safety, toxicology, and GLP-compliant studies supporting IND submissions.
- Assay & Biomarker Development – Specialized expertise in custom assay design, biomarker validation, and molecular and immunological profiling.
- Translational Precision Medicine – Proprietary platforms for patient stratification, biomarker-based validation, and AI-enhanced predictive modeling.
- Flexible Delivery Models – Modular or integrated support models including FFS, FTE, and milestone-based engagements tailored to program needs.
- Quality & Compliance – Rigorous GLP standards and seamless data integration ensure regulatory-ready deliverables and reproducibility across discovery and preclinical phases.
This structure allows Genovyn and its subsidiaries to advance novel compounds from concept to IND-ready status with exceptional scientific continuity and speed.

Clinical, Regulatory & Commercial Execution — Leadership-Driven
Genovyn’s leadership team brings decades of experience across the global clinical research and commercialization ecosystem, having held senior positions at leading Contract Research Organizations (CROs).
Collectively, the team has overseen hundreds of clinical studies worldwide, supporting both major pharmaceutical companies and emerging biotech innovators. Their experience spans every phase of clinical and commercial development — from first-in-human trials through global Phase III programs and successful product launches.
Clinical Operations Leadership
- Deep operational experience in Phase I–IV program execution, adaptive trial design, site activation, and patient recruitment across multiple therapeutic areas.
- Regulatory Oversight – Proven track record navigating FDA, EMA, and ICH frameworks with emphasis on quality, pharmacovigilance, and submission readiness.
- Patient Recruitment & Site Networks – Longstanding relationships with global investigators and CRO networks to accelerate enrollment and enhance diversity in clinical studies.
- Technology & Data Integration – Familiarity with AI-driven data platforms, eSource technologies, and real-world evidence tools adopted across major CROs.
- Commercial Strategy & Market Access – Expertise in launch planning, pricing and reimbursement strategy, lifecycle management, and market access optimization.
- Leveraging Deep Industry Expertise
Rather than maintaining in-house clinical operations, Genovyn leverages its leadership’s extensive industry experience and preferred provider network to identify, select, and manage the most effective global CRO partners for each program.

Why Genovyn’s Integrated Model Matters
Tomorrow’s therapies demand a new kind of collaboration. Genvoyn’s integrated model drives alignment across science, data, and development to redefine what’s possible in disease care.
- Seamless Continuity — Unified control from target discovery through commercialization ensures faster development and fewer handoffs.
- Capital Efficiency — Shared infrastructure, CRO partnerships, and internal oversight reduce overhead and preserve investor capital.
- Risk Mitigation — IP control, milestone-based oversight, and quality audits protect both science and shareholder value.
- Strategic Flexibility — Our model allows modular participation — integrating with third-party CROs when required by investors, while maintaining TheraIndx’s scientific continuity.

The Genovyn Difference
Genovyn’s model fuses the agility of a biotech incubator with the rigor of a global CRO. By aligning our deep discovery science from Genovyn’s clinical and commercial leadership, we deliver a scalable, efficient path from innovation to market — accelerating therapies that improve patient outcomes and investor returns alike.

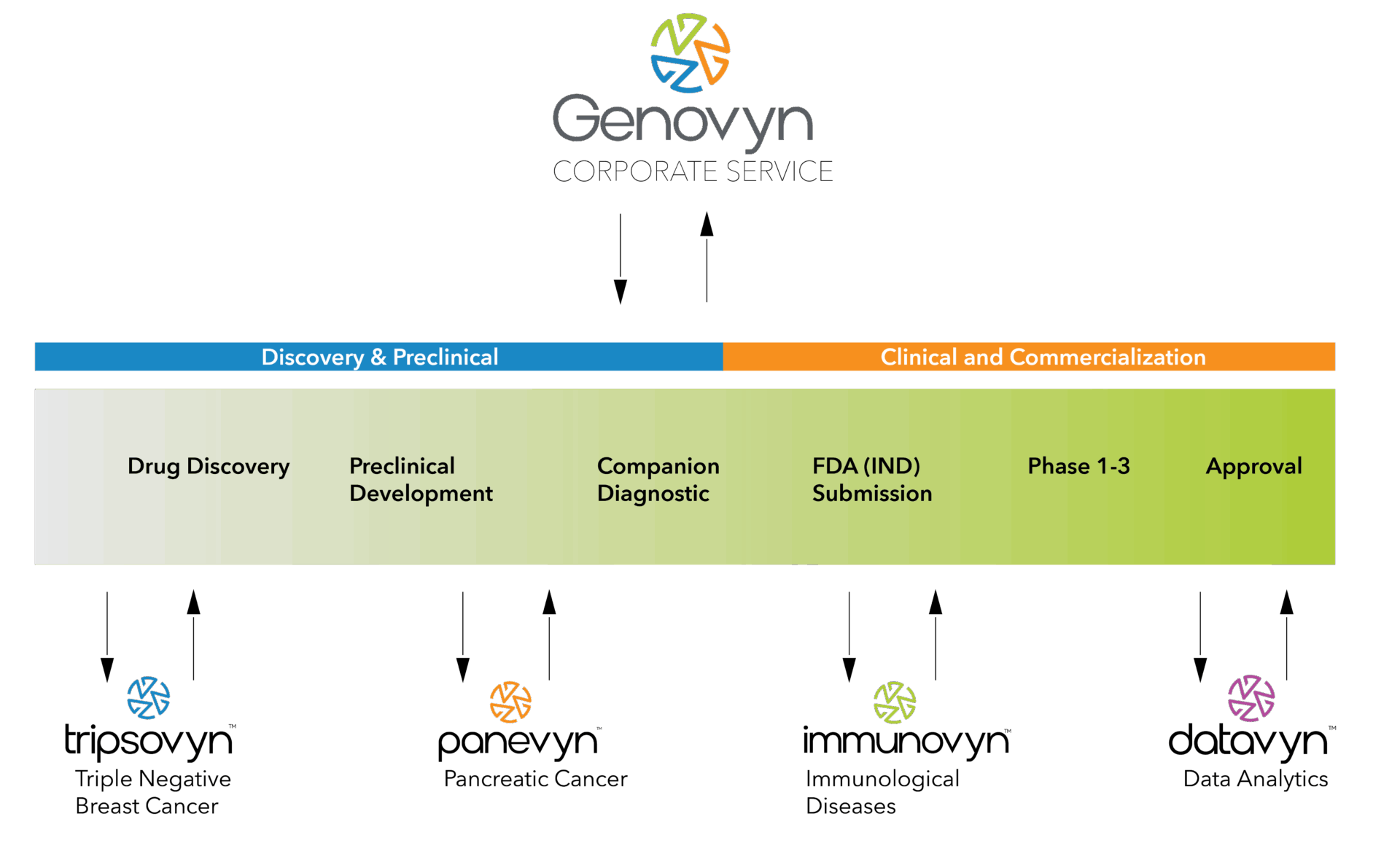
Our Business Model
Genovyn’s integrated discovery and development model enhances program success rates while reducing both time and cost to market. Our portfolio companies—known as “Vyns”—are therapeutically focused and operationally agile, leveraging the centralized infrastructure, resources, and expertise of the Genovyn platform.
Each Vyn is led by seasoned domain experts aligned around a clear therapeutic mission, combining scientific rigor with strategic execution. This structure enables Genovyn to scale innovation efficiently across diverse therapeutic areas while maintaining focus, speed, and capital discipline.